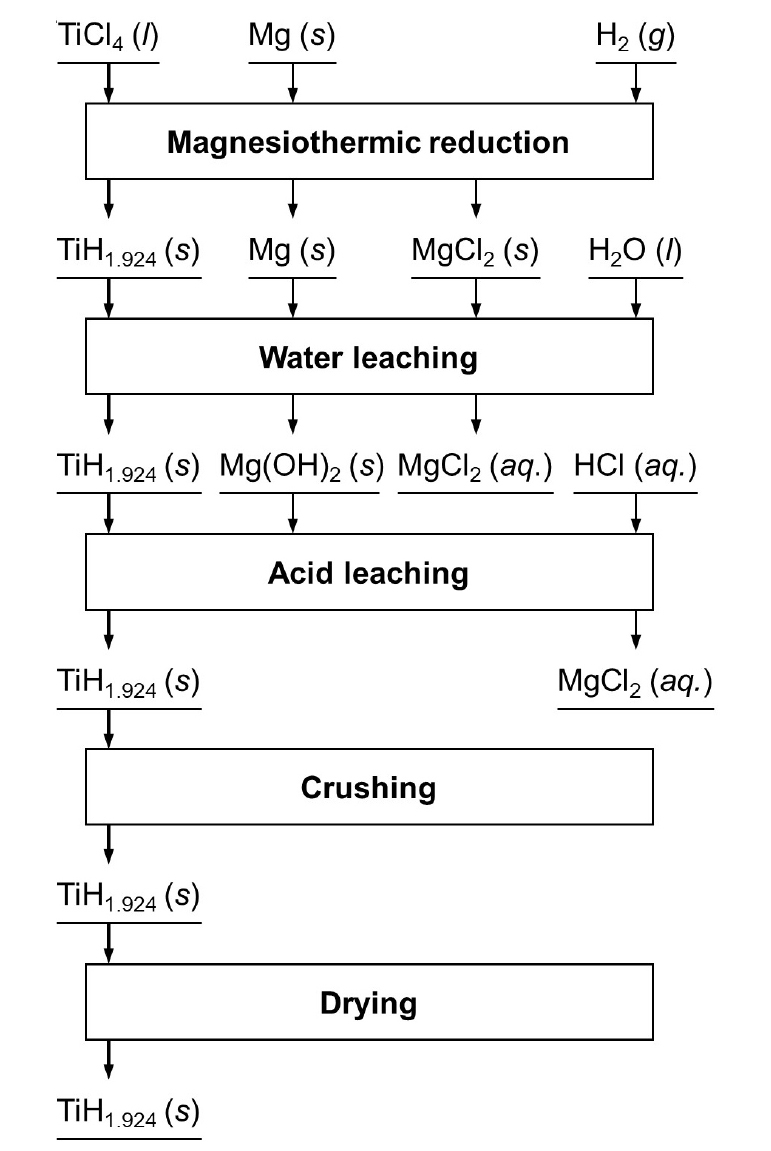
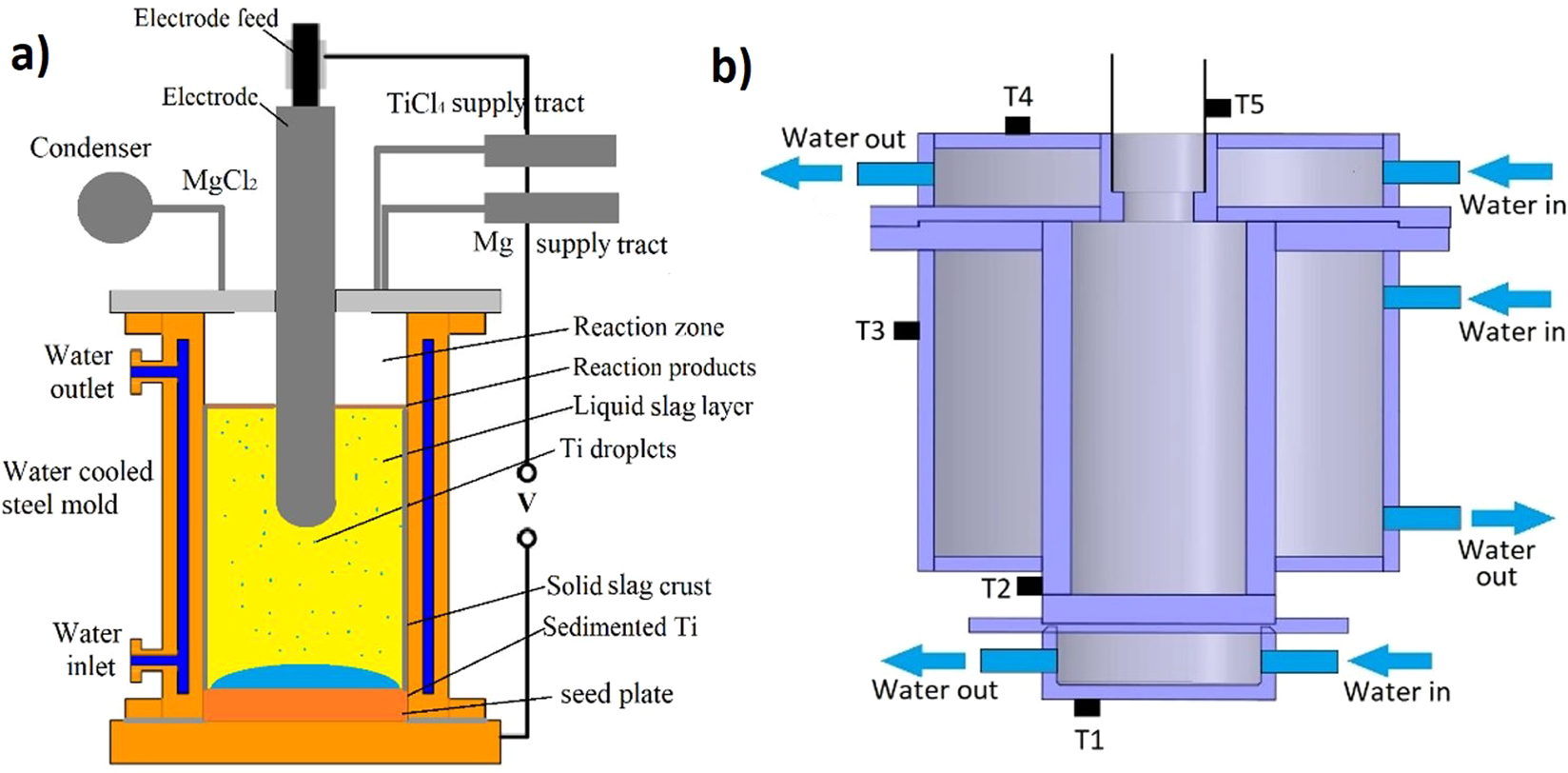
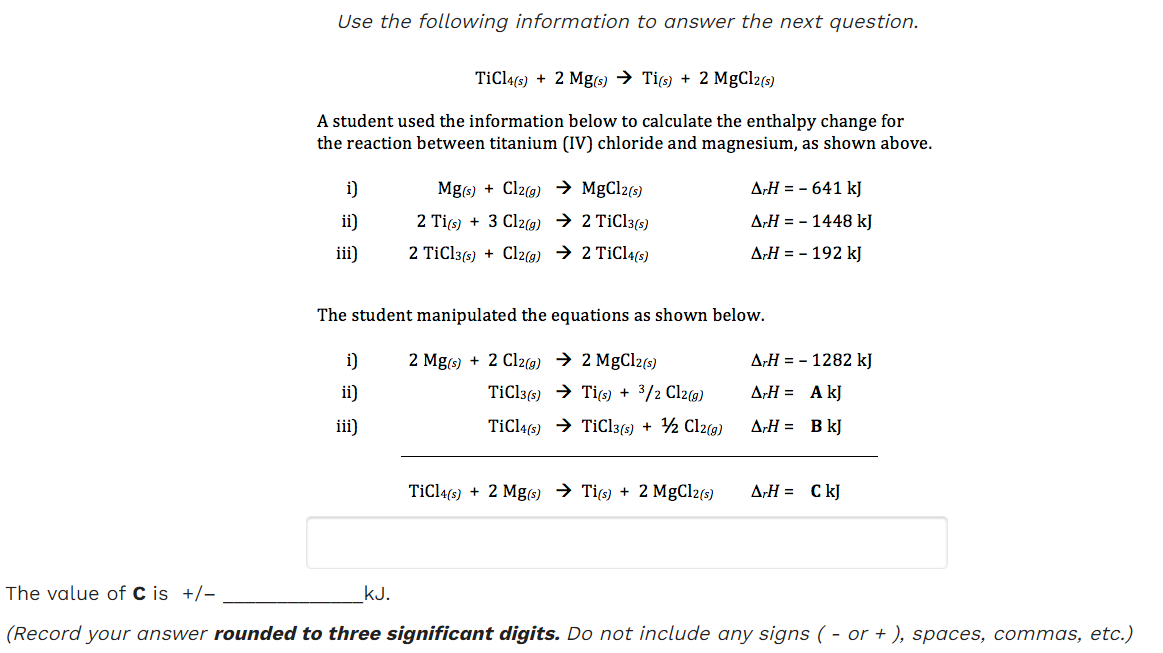
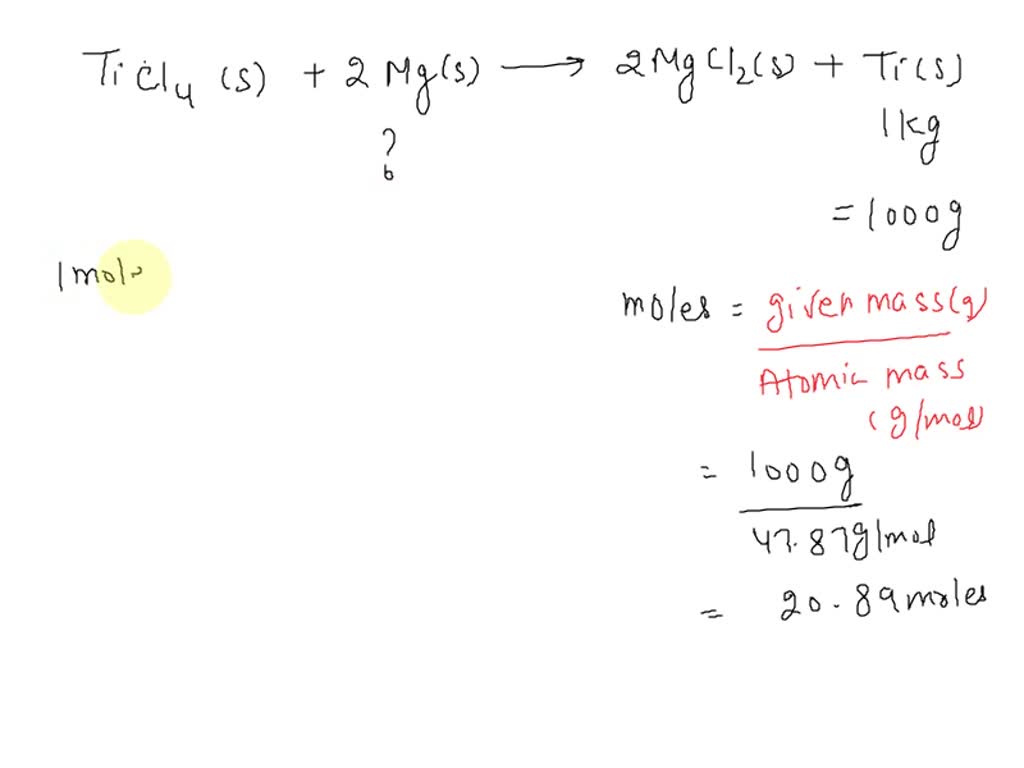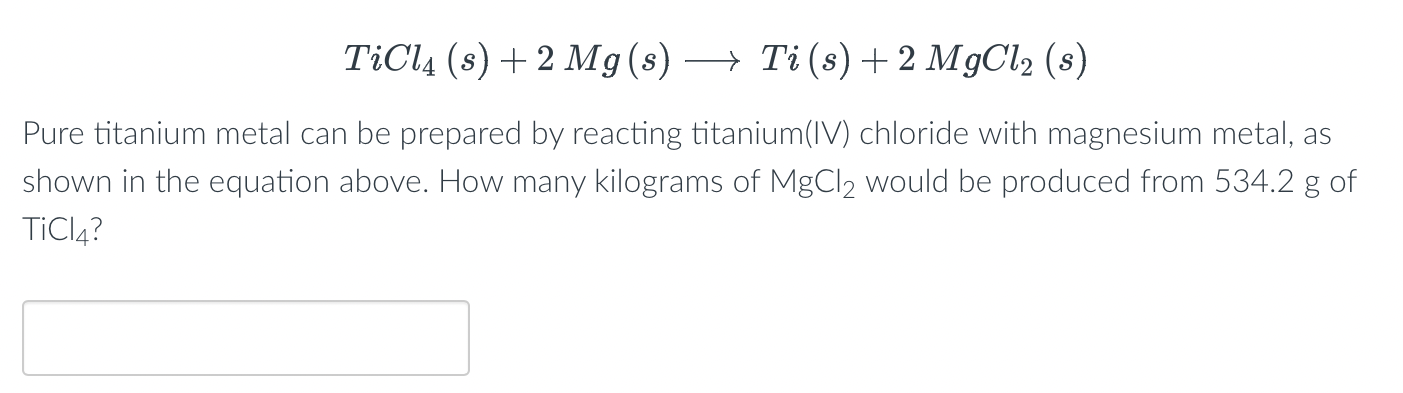PDF) Study of Ethylene Polymerization Kinetics by TiCl4/Mg(OC2H5)2 Catalyst and Determination of the Active Sites Concentration | SHOKOUFEH HAKIM - Academia.edu

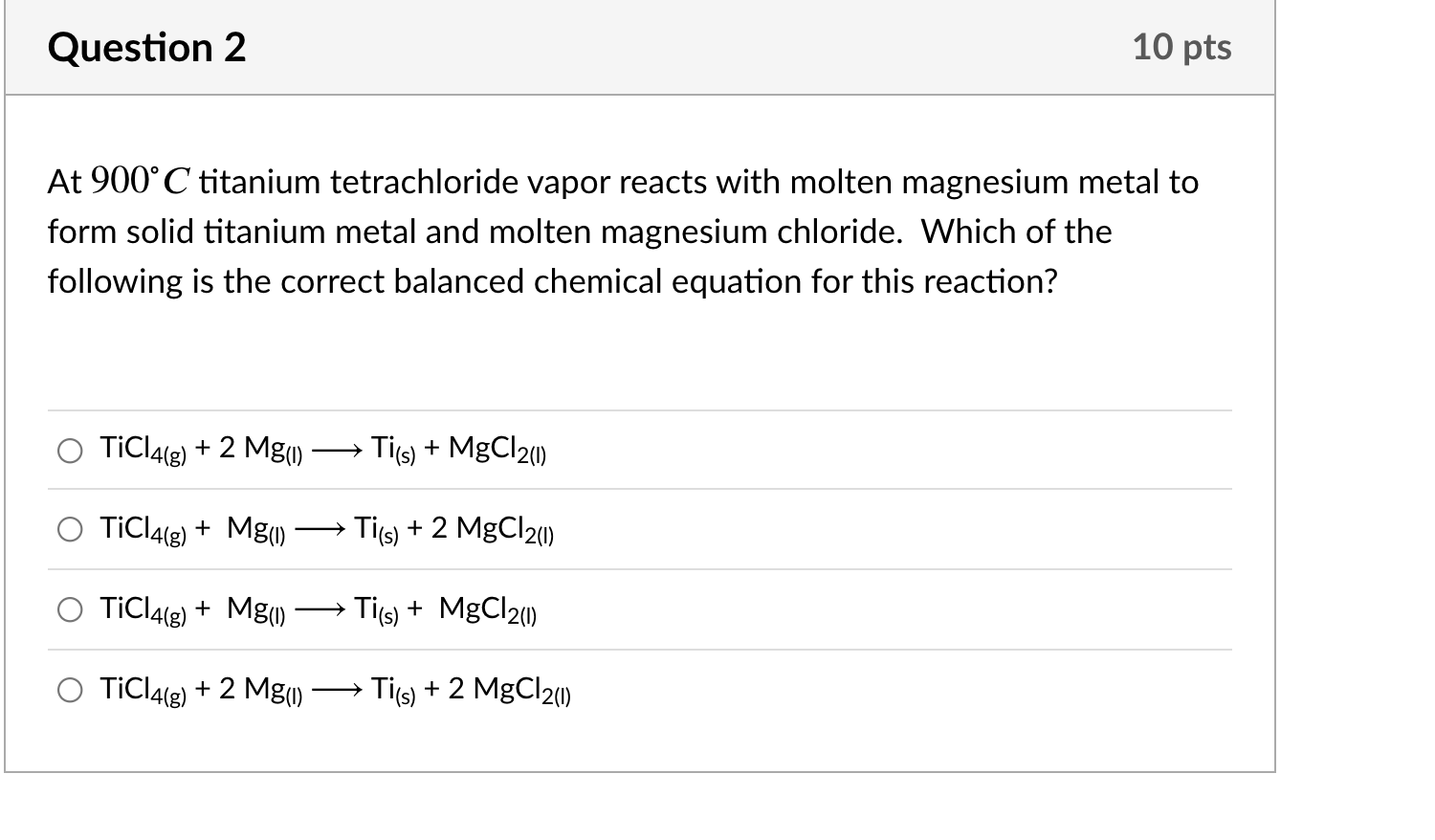
TiCl4(l) + Mg(s) → Ti(s) + MgCl2(s) Balance the following chemical equations and identify the type - YouTube

Bromoform Activation. TiCl4–Mg-Promoted CHBr2– and CBr3– Transfer to a Variety of Aldehydes and Ketones | Organic Letters

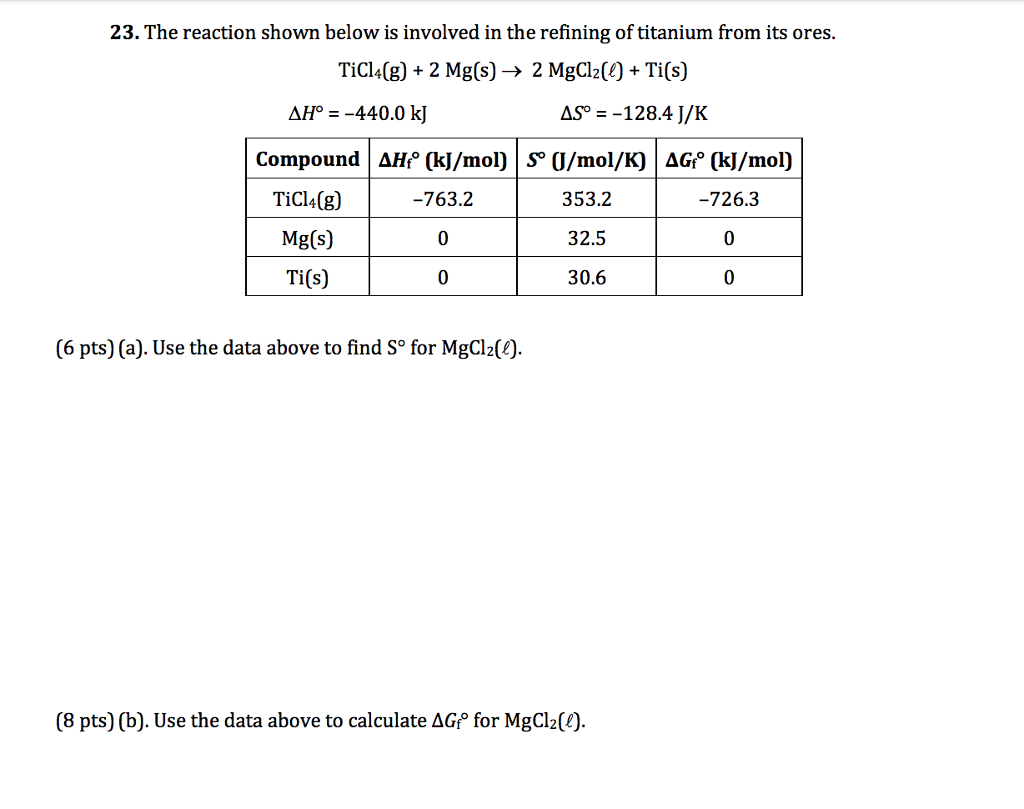
TITUCII AIC13 van levretically IUIIII! (HS . C12, 1.75 27) In the manufacture of titanium, what mass of Ti can theoretically be formed when 1 kg of TiCl4 reacts with 0.1 kg

New, general, and practical preparation of methyl ketones via the direct coupling of amides with CH2Cl2 promoted by TiCl4/Mg. | Semantic Scholar

Alkylation of titanium tetrachloride on magnesium dichloride in the presence of Lewis bases - ScienceDirect

X-ray diffraction patterns of the titanium sponge with TiCl4 feeding... | Download Scientific Diagram